Fugitive Greenhouse Gas Emissions in a Global Hydrogen Economy
A 2022 report commissioned by the Department for Business, Energy & Industrial Strategy in the UK considered the possible implications of fugitive hydrogen (H2) emissions from an illustrative future scenario where widespread use of H2 has been adopted globally. In this scenario, they assumed that set percentages of the final energy consumption in specified energy sectors, currently supplied by fossil fuel, switch to H2.
The scenarios modelled for different amounts of fugitive H2 leakage indicate that H2 will affect the concentration of methane, ozone, and water vapor in the atmosphere. The changes in methane and ozone are driven by changes in the hydroxyl radical, OH, which is the major atmospheric oxidant and a key player in the chemistry of the atmosphere. Modelled changes in radiative forcing, like the modelled changes in atmospheric composition, indicate that, to maximize the climate and air quality benefit of a transition to a hydrogen-powered economy, minimization of both fugitive hydrogen leakage and a reduction of the ancillary emissions of, for example, CO, NOX, and VOCs is required.
Introduction
Greenhouse Gases
The lowest portion of the atmosphere is the troposphere, a layer where temperature generally decreases with height. This layer contains most of Earth’s clouds and is the location where weather primarily occurs.
The layers of Earth's atmosphere, with a yellow line showing the air temperature at various heights. Encyclopædia Britannica.The lower levels of the troposphere are usually strongly influenced by Earth’s surface. This sublayer, known as the planetary boundary layer, is that region of the atmosphere in which the surface influences temperature, moisture, and wind velocity through the turbulent transfer of mass.
Generally confined within the troposphere, a “greenhouse gas” is a gas that absorbs and emits radiant energy within the thermal infrared range, causing the greenhouse effect. Without greenhouse gases, Earth’s surface temperature would be about 33 degrees Celcius colder.
The principal greenhouse gases are water vapor (H2O), carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), ozone (O3), and fluorinated gases (hydrofluorocarbons, HFCs; perfluorocarbons, PFCs; sulfur hexafluoride, SF6; nitrogen trifluoride, NF3).
Excluding water vapor, the other greenhouse gases constitute about 0.05% of Earth’s total atmosphere. Each gas is also non-condensable, so as atmospheric temperatures change, they cannot be converted to liquid form, and their concentration remains stable. Furthermore, CH4 and CO2 are not particularly chemically reactive or easily broken down by light in the troposphere.
Two concepts used frequently in discussions of the greenhouse effect are “Radiative forcing” (or climate forcing) and the “Global warming potential” (GWP) of a greenhouse gas.
Radiative forcing is the change in energy flux in the atmosphere caused by natural or anthropogenic factors of climate change. Positive radiative forcing means Earth receives more incoming energy from sunlight than it radiates to space. This net gain of energy will cause warming. Conversely, negative radiative forcing means that Earth loses more energy to space than it receives from the sun, which produces cooling.
The GWP is defined as the cumulative radiative forcing over a specified time horizon resulting from the emission of a unit mass of gas relative to a reference gas (CO2). GWP is 1 for CO2. For other gases it depends on the gas and the time frame. Over a 100-year timeframe, the GWC for methane is 25, for N2O it is 298, and although it is negligible for water vapor other factors mentioned below are relevant. [https://en.wikipedia.org/wiki/Global_warming_potential]
Water vapor is Earth’s most abundant greenhouse gas, and because it is condensable, its concentration depends upon the temperature of the atmosphere. Most public attention in recent years has understandably been on the greenhouse gases of anthropogenic origin, but little attention is given to the role of water vapor. Increased atmospheric temperature, generally attributed to increased anthropogenic emissions of greenhouse gases, leads to increased concentration of water vapor and a supercharging of the warming caused by the other greenhouse gases.
At the start of the cycle, increased temperatures lead to increased evaporation from both water and land areas. Because warmer air holds more moisture, the concentration of water vapor increases.
Specifically, this happens because water vapor does not condense and precipitate out of the atmosphere as easily as at high temperatures. The water vapor then absorbs the heat radiated from the Earth, thereby contributing to a positive feedback loop, and significantly increasing the warming that would occur from increasing carbon dioxide alone.
Report Assumptions and Scenarios
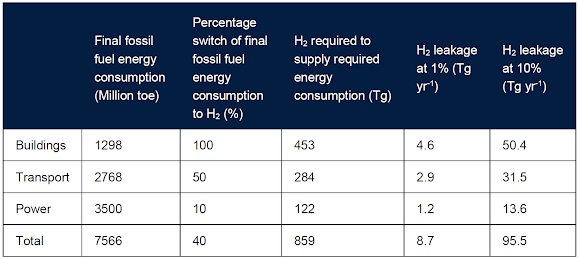
The amounts of fugitive H2 emissions resulting from a hydrogen economy will depend on the level of H2 usage in different energy sectors and the percentage of H2 that leaks to the atmosphere during its production, transport, and storage.
The BEIS report estimated fugitive H2 emissions from an illustrative future scenario where widespread use of H2 has been adopted globally. In this scenario, they assumed that set percentages of the final energy consumption in specified energy sectors, currently supplied by fossil fuel, switch to H2.
Estimates of potential fugitive H2 leakage in a global hydrogen economy. 1 Tg = 1 million tonnes.
H2 is currently present in the atmosphere with a mixing ratio of about 0.55 parts per million (ppm). Present day sources of H2 include fossil fuel combustion and biomass burning which are estimated to represent approximately 50% of the total global H2 source (about 20-25% from fossil fuel combustion), with the remainder arising from the oxidation of CH4 and volatile organic compounds (VOCs) in the atmosphere (note: no consideration given for natural hydrogen emissions).
Any decreases in H2 emissions resulting from a reduction in fossil fuel combustion may therefore partially offset any increases in H2 emissions resulting from fugitive H2 leakage in a hydrogen economy. Furthermore, they assumed that H2 will be generated using a production method with no associated upstream CH4 emissions, and CO2 is fixed in their model simulations (so changes to CO2 emissions are also not considered).
Today, the majority of H2 is generated from steam methane reformation (SMR) which is associated with emissions of both CH4 and CO2 (unless CO2 capture, and storage is utilized). Atmospheric H2 is removed primarily by uptake to soils, but also through reaction with hydroxy radicals (OH) in the atmosphere (discussed also below).
Fugitive H2 leakage rates are likely to be higher than for natural gas owing to the small molecule size of H2.
The BEIS report notes a recent study looking at the US natural gas supply chain indicated natural gas leaks of around 2.3% of gross gas production. The BEIS report considered a range of possible hydrogen future global scenarios, with surface mixing ratio increases ranging from 0.25 parts per million (50% increase) by volume (ppm or millimoles/mole) to 1.5 ppm (300% increase) above the current background mixing ratio of about 0.5 ppm.
They believe these scenarios span much of the uncertainty in potential changes to the atmospheric mixing ratios of H2 associated with the ultimate size of the hydrogen economy, the H2 soil sink and H2 leakage rates.
For example, taking a hydrogen economy of the size required to supply 23% of present-day global energy consumption, and assuming the magnitude of the soil sink increases in line with the increase in H2 mixing ratios (i.e., a constant deposition velocity), simulations indicate H2 boundary conditions of 0.75, 1, 1.5 and 2 ppm represent H2 leakage rates of about 3, 7, 13% and 20% respectively.
Their 2 ppm H2 scenario should be considered as an extreme end member designed to assess the linearity of the atmospheric response to increasing atmospheric H2, rather than a prediction of potential future atmospheric H2 levels in an H2 economy.
When combined with the proposed global widespread H2-use, their upper leakage rate of 10% represents a likely upper limit on the increase in atmospheric H2 associated with a hydrogen economy. A leakage rate of 10% would be likely to be both unsafe and expensive, leading to pressure for development of better containment.
They also considered the impact of changes in emissions of gases other than CO2 which could follow increased adoption of H2 as an energy source; these gases, emitted alongside CO2 and called hereafter ‘co-emitted species’, include carbon monoxide (CO), CH4, VOCs and the oxides of nitrogen (NOX).
Report Findings and Implications
Fugitive hydrogen leakage will affect the concentration of CH4, ozone, and water vapor in the atmosphere. The changes in methane and ozone are driven by changes in the hydroxyl radical, OH, which is the major atmospheric oxidant and a key player in the chemistry of the atmosphere. H2 acts as a chemical sink for OH, and so increases in hydrogen concentrations lead to a reduction in tropospheric OH:
H2 + OH = H2O + H (1)
Another important consequence of any increase in atmospheric H2, and the subsequent decrease in OH, would be to increase the lifetime of CH4, which is largely controlled by the reaction with OH:
CH4 + OH = CH3 + H2O (2)
Methane is second only to CO2 in its impact as an anthropogenic greenhouse gas; lengthening the lifetime of CH4 increases its radiative forcing (for a given CH4 emission) and global warming potential (GWP). So, emissions of H2 into the atmosphere would contribute indirectly to radiative forcing; H2 is an indirect greenhouse gas.
The BEIS report concludes that if methane emissions remain constant, increased hydrogen emissions would result in a longer methane lifetime and a higher methane abundance.
When reductions in emissions of CO2, oxides of nitrogen (NOX), and volatile organic compounds (VOCs) are also considered alongside increases to atmospheric hydrogen, the decrease in OH, and hence increase in CH4, is smaller.
The response of CH4 to a shift to a hydrogen economy is therefore complex and strongly scenario dependent. Under the maximum increase of 1.5 ppm of hydrogen considered, to offset the potential increase in methane would require further decreases in not just emissions of methane, but also the co-emitted species of carbon monoxide, oxides of nitrogen and volatile organic compounds.
As observed in (2), changes in H2 will also affect atmospheric water vapor.
The H2O abundance in the troposphere is dominated by the hydrological cycle (evaporation from the oceans, precipitation, etc.) but increases in atmospheric H2 will likely lead to an increase in stratospheric water vapor and the associated radiative impacts. As an example of the modelling performed, Figure 2 shows the zonal mean water vapor response to increases in atmospheric H2. The impact from chemical reactions on tropospheric water vapor is ignored. It is observed in Figure 2 that stratospheric water vapor mixing ratios increase with increasing H2, with increases of up to 25% in the (upper) scenario of 1.5 ppm H2 increase above the current background mixing ratio of about 0.5 ppm
The response of tropospheric ozone is complex and depends not simply on the changes in hydrogen abundance but also on changes in the emissions of other species. Stratospheric ozone is controlled by different chemical processes to those in the troposphere, but for the range of calculations performed, no discernible negative impacts on global stratospheric ozone recovery were modelled.
The BEIS report estimates the H2 GWP over a 100-year timeframe to be 11 ± 5; a value more than 100% larger than previously published calculations.
About a third of this arises from the changes in stratospheric water vapor that follow from an increase in atmospheric H2.
Most of the uncertainty in the GWP arises from uncertainty about the natural budget of atmospheric H2, where the magnitude of the soil sink for H2 is the most uncertain factor. Example modelled equilibrium global-mean temperature changes are shown below for different emissions scenarios.
The net top-of-atmosphere radiative forcing varies strongly regionally. It depends in a complex fashion on the changes in gas phase composition, the subsequent impact on aerosol production and on cloud and aerosol interactions.
The changes in radiative forcing, like the changes in atmospheric composition, indicate that, to maximize the climate and air quality benefit of a transition to a hydrogen-powered economy, minimization of both H2 leakage and a reduction of the ancillary emissions of, for example, CO, NOX, and VOCs is required.
Global zonal mean H2O mixing ratios for the background simulation of H2 concentration (A), and percentage differences with respect to the background simulation for 0.75 ppm H2 (B), 1 ppm H2 simulation (C), and 2 ppm H2 simulation (D). Results shown are averages from the final 25 years of the model simulations; only differences that are statistically significant at the 95% confidence level are presented. Vertical axis is altitude above MSL and horizontal axis is latitude (0 = equator).
Summary
Climate modelling based upon fugitive emissions is clearly complex and must make many assumptions. No opinion or endorsement is made here regarding the veracity of the published results, but the implications for how fugitive H2 might interact with greenhouse gases in the troposphere and stratosphere is interesting.
From the perspective of the maritime industry (and marine seismic surveys), until H2 bunker fuel is commercially practical on a global scale the fuel cannot be considered.
It correspondingly seems likely that incentives to transition to such fuels in a hydrogen economy are likely to be governed by additional regulations regarding how H2 is produced, transported, stored, and utilized.
Disclaimer
The content discussed here represents the opinion of Andrew Long only and may not be indicative of the opinions of Petroleum Geophysical AS or its affiliates ("PGS") or any other entity. Furthermore, material presented here is subject to copyright by Andrew Long, PGS, or other owners (with permission), and no content shall be used anywhere else without explicit permission. The content of this website is for general information purposes only and should not be used for making any business, technical or other decisions.







Comments
Post a Comment